The number of Advanced Therapy Medicinal Products (ATMPs) approved by the Medicines and Healthcare products Regulatory Agency (MHRA) is expected to rise significantly in the coming years. The MHRA has only approved an average of two ATMPs annually but the UK is leading the way with clinical trials in this area despite uncertainty remaining about how long the benefits of ATMPs might persist. The potential benefits are such that the investment is vital. AMTPs including cell and gene therapies offer hope for diseases previously considered untreatable. ATMPs are already being used to treat some rare conditions, including haemophilia and spinal muscular atrophy. In some cases, these therapies can transform people’s lives with just a single treatment. Therapies now in development are aiming to address conditions that affect larger patient populations, including certain types of dementia and Parkinson’s disease.
Informed Insurance
Informed Insurance

Scenario planning in 2025 - In the grip of uncertainty
We are now all operating outside the previous boundaries of our experience, buffeted by forces we cannot predict, let alone control or influence. I...

The ESG "backlash": How to balance competing demands
Climate change and social issues have, for (at least) the last decade, occupied legislative, regulatory and shareholder thinking, with implications...
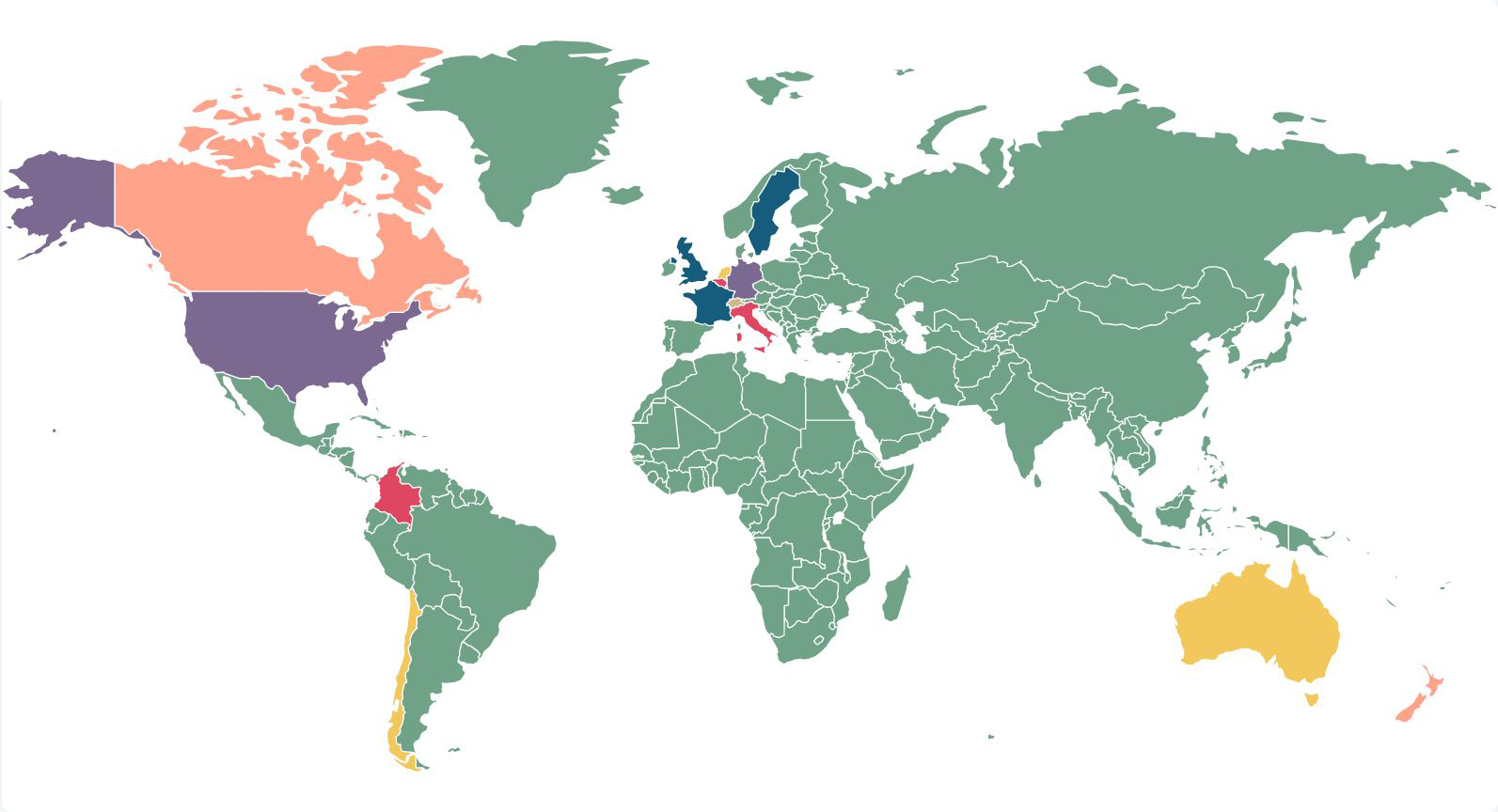
Developments in climate change litigation: 2025 could herald an expansion in types of claim and remedies
To accompany the fourth edition of our interactive climate change litigation map, we take a deeper dive into the implications of the key decisions ...

Our Top Five Economic predictions for 2025
To highlight our new economic theme on Informed Insurance, Charlotte Shakespeare, Legal Director and editor of our Predictions for 2025, shares her...

Our Top Five ESG predictions for 2025
In our ongoing #DACBCrystalBall series, Charlotte Shakespeare, Legal Director and editor of our Predictions for 2025, shares her Top Five ESG predi...

Predictions 2024 – Charlotte Shakespeare's Top Ten
In our ongoing #DACrystalBall series, Charlotte Shakespeare, Legal Director and editor of our Predictions for 2024, once again shares her Top Ten p...